Difference between Liquid Chromatography and Gas Chromatography
Chromatography is a process for separating components of a mixture for analysis. This is a component of our small molecule analysis testing and plays a critical part in Identifying and quantifying impurities. It is also crucial in screening raw materials for identification, to ensure the quality and safety of products, as well as evaluate reactions by looking at residual analytes to better understand how a product will interact with other components in the supply chain.
Material purity is being pushed to unprecedented levels in many different industries. Whether it’s to extend the life of electronic products or ensure the efficacy of pharmaceutical drugs, being able to report the impurities with a part-per-trillion detection capability should be an expected outcome of any small molecule analysis test, and chromatography allows us to achieve that.
Identifying and quantifying impurities are among the main benefits for conducting chromatography. Screening raw materials for fingerprinting, tracking reactions, and understanding material impurities allows you to evaluate reactions by looking at residual analytes. Screening raw materials is a crucial part of Brewer Science’s metrology process, and a key factor in our Zero Defects Program. The actual process involves separating different components through the chromatography method most appropriate to the mixture, as explained further in the method section. We can then use analytical chromatography to conduct qualitative and quantitative analysis of the components of a mixture.
Brewer Science offers many different analytical and application testing services, however, understanding the difference between liquid chromatography and gas chromatography will allow you to visualize the opportunity that exists in analytical testing. Speak with a small molecule analysis expert to understand which small molecule analysis is best for you.
Gas chromatography (GC) and high-performance liquid chromatography (HPLC) are both staple techniques for separations used in our small molecule analysis lab. These differences are also summarized in the chart.
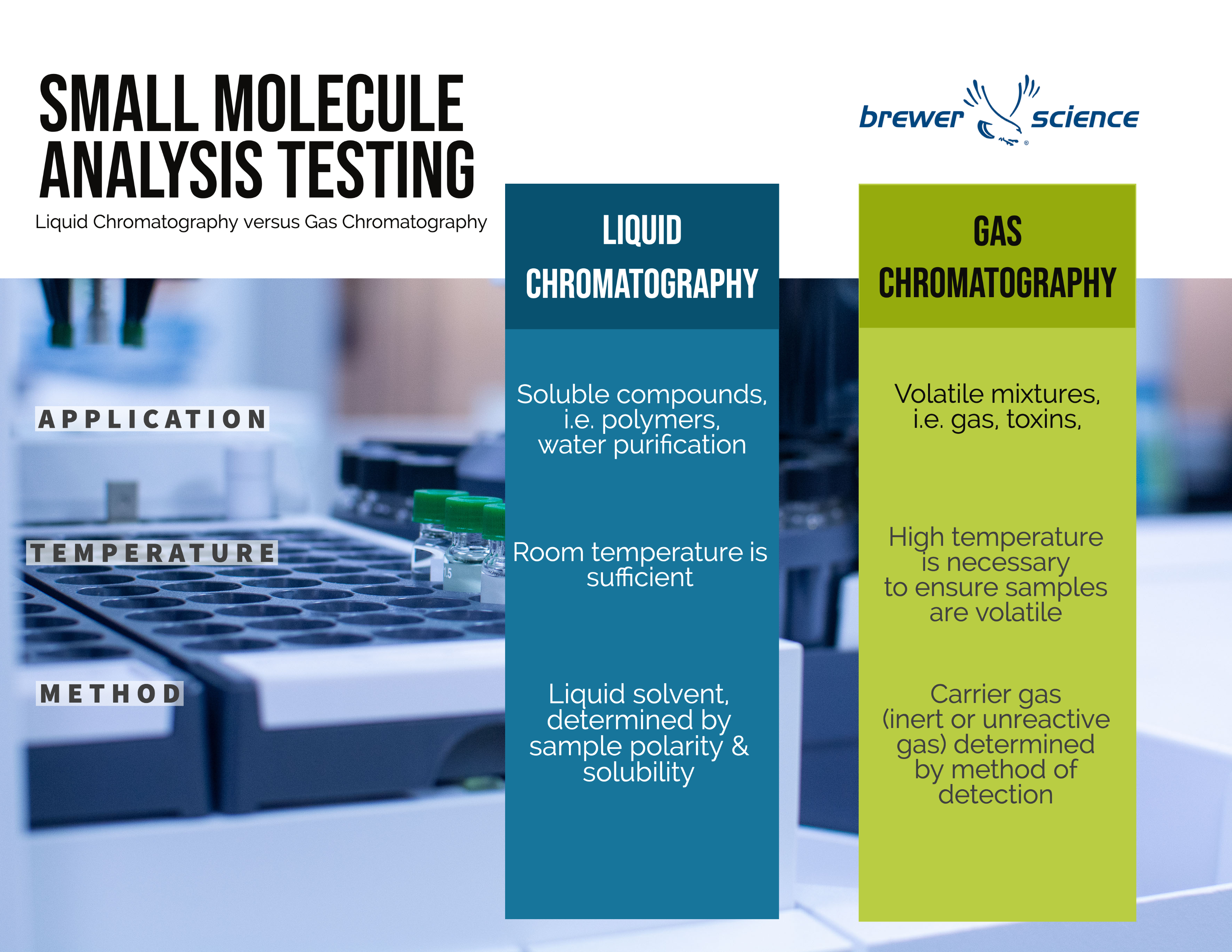
Method
The main difference between gas chromatography and high-performance liquid chromatography is the mobile phase used. HPLC uses a liquid solvent, which is determined by the complexity of the compounds in the sample alongside their polarity and solubility. GC uses an inert or unreactive gas, known as a carrier gas, which is determined on the subsequent method of detection
Temperature and Speed
High-performance liquid chromatography can be performed at room temperature. In contrast, gas chromatography requires a much higher temperature to ensure samples are volatile. Because of this volatility, however, GC is much quicker to separate molecules than HPLC. Volatile compounds can move through the system in minutes or even seconds, compared to HPLC runs that are generally between 10 and 60 minutes. GC is used for volatile compounds while HPLC is better for less volatile samples. If a sample contains salts or carries a charge, it must be analyzed using HPLC, not GC. 
Applications
HPLC is suitable for analyzing soluble compounds, making it highly useful for food substances, water purification and polymers. It relies on pumps to pass a pressurized liquid solvent containing the sample mixture through a column filled with a solid adsorbent material .
GC requires a volatile mixture, typically a gas. As a result, it’s used for things like drugs, toxins and air samples. With GC, the sample solution injected into the instrument enters a gas stream which transports the sample into a separation tube known as the “column.” Helium or nitrogen is often used as the carrier gas. The various components are separated inside the column.
Flame ionization detection is the most used GC detection method due to its superior ability to measure hydrocarbons. Using a carrier gas, the sample is ionized via a hydrogen-air flame, then collected using a polarizing voltage. The current produced is measured and used to quantify the amount of sample being burned. Additionally, GC can be coupled with Fourier transform IR (GC-FTIR). Proper analysis of a small molecule first requires separating it and then identifying its properties. Therefore, the two methods are frequently coupled in an acronym, such as (LC-MS), which stands for liquid chromatography (separation technique), and mass spectrometry (identification). 
Mass spectrometry is an analytical tool useful for measuring the mass-to-charge ratio (m/z) of one or more molecules present in a sample. These measurements can often be used to calculate the exact molecular weight of the sample components as well.
Brewer Science performs GC-MS and GC-FTIR, in additional to a complete list of other analytical and testing capabilities.
Equipment and cost
The equipment used for GC versus HPLC is different, since it requires different methods to carry out the mobile phase. Therefore, there is a significant cost difference between the two techniques.
Columns used for GC are long and thin, while HPLC columns are shorter and wider. HPLC also requires expensive solvents and a pressure pump to push the mobile phase through the column. On the other hand, gas chromatography simply requires gas containers and carrier gas, which is more affordable than solvents. Thus, GC is generally seen as the more cost-effective option. 
Understanding the fundamental differences between the two main molecule separation methods, gas chromatography, and high-performance liquid chromatography will aid your decision in determining which testing methods will best serve your business and customers. Small Molecule Analysis testing has been part of the many testing services Brewer Science performs to uphold the Zero Defects Program. With over four decades of analyzing small molecules and perfecting our metrology, we recently announced we are offering stand-alone testing services to customers across the globe to further push material purity to higher expectations.
Schedule a call with a testing services expert to explore our complete line of analytical and application testing services, specializing in semiconductor and other high-technology products, through materials characterization and contaminant reduction.

